NIHR Lancashire Clinical Research Facility

Our vision
To facilitate experimental medicine in Lancashire and South Cumbria, enabling all people in our community to take part in research studies.
The National Institute for Health and Care Research (NIHR) Lancashire Clinical Research Facility (CRF) is part of the NIHR and hosted by Lancashire Teaching Hospitals NHS Foundation Trust.
The NIHR Lancashire CRF supports and conducts clinical research studies and is based in a purpose-built unit within the Avondale Unit, Lancashire Teaching Hospitals NHS Foundation Trust (Royal Preston Hospital site).
We have highly trained nurses, scientists, doctors and support staff dedicated to research. Our outstanding research facilities include a bespoke ward and consulting rooms, a laboratory and specialist equipment.
The NIHR Lancashire CRF aims to improve patient care by increasing understanding of the causes of disease and improving diagnosis and treatment. Our collaborative and centralised approach maximises efficiency and ensures the patient voice is central in clinical research.
Read our latest STAR Quality Assurance accreditation report here.
Telephone: 01772 522031
Email: LancashireCRF@LTHTR.nhs.uk
- About Us
The NIHR funds, enables and delivers world-leading health and social care research that improves people's health and wellbeing and promotes economic growth. NIHR Clinical Research Facilities (CRFs) support the delivery of early-phase and complex studies in purpose built facilities in NHS hospitals. The NIHR has awarded £161 million over five years to 28 CRFs across England.
The NIHR Lancashire CRF is a partnership between Lancashire Teaching Hospitals NHS Foundation Trust, Lancashire & South Cumbria NHS Foundation Trust, the University of Central Lancashire, and Lancaster University. Together the teams provide a unique combination of both physical and mental health research expertise. We conduct research in a wide variety of medical fields, however our priority areas are Cancer, Neurology (including Dementia) and Mental Health.
We aim to improve patient care by increasing understanding of the causes of disease and improving diagnosis and treatment.
- Our goal is to support new and established investigators in Lancashire and South Cumbria in developing and delivering clinical research.
- We maintain a strong relationship with Liverpool, Manchester and Sheffield NIHR CRFs being members of the NIHR North-West CRF Alliance and the UK CRF Network.
- We are preferred partners to several Clinical Research Organisations (CROs).
Our research has been published in top journals and received national awards and recognition.
- News and events
- Examples of Trials
AGILE CST-2 study (COVID19)
.png) As part of the national response to the SARS-CoV-2 (COVID-19) pandemic, the NIHR Lancashire CRF was involved in a number of clinical trials assessing the efficacy and safety of treatment options.
As part of the national response to the SARS-CoV-2 (COVID-19) pandemic, the NIHR Lancashire CRF was involved in a number of clinical trials assessing the efficacy and safety of treatment options.One such trial was the AGILE platform trial. In AGILE CST-2 the CRF treated COVID19 positive patients with either an anti-viral drug called Mulnupiravir or a placebo. Patients were followed up with routine visits for up to one month to assess symptoms and if they are improving. This trial was reported on ITV news.
PERSICA-002 study (Back Pain)
.png) Low back pain is a common and often debilitating condition for patients. The NIHR Lancashire CRF was one of a few select sites worldwide involved in the early phase PERSICA-002 Part A trial using an antibiotic injection around the discs of the spine. Patients were reviewed at outpatient appointments for up to one year to monitor the effects. This study has now moved to the next stage offering two injections with to monitor the beneficial effect this may have on patients.
Low back pain is a common and often debilitating condition for patients. The NIHR Lancashire CRF was one of a few select sites worldwide involved in the early phase PERSICA-002 Part A trial using an antibiotic injection around the discs of the spine. Patients were reviewed at outpatient appointments for up to one year to monitor the effects. This study has now moved to the next stage offering two injections with to monitor the beneficial effect this may have on patients.If you are interested in part B of this study, please contact us via phone 01772 522031 or email us LancashireCRF@lthtr.nhs.uk
Peter Hamlyn, Consultant Spinal Neurosurgeon, Co-Founder of Persica “After a lifetime searching for a treatment that would definitively relieve my patients’ suffering, the scientific team at Persica have delivered a World first in a therapy, relevant to a huge number of these patients, that genuinely offers to cure the underlying cause of their problem.”
GALAHAD (Cancer)
.jpg) The Galahad trial was aimed at men with Prostate Cancer that had spread throughout the body (metastasised) who had specific anomalies in their DNA. Patients took the trial drug Niraparib as a tablet with regular visits to the CRF for blood tests and physical exam to check the effect the drug was having and any possible side effects. Regular imaging scans were arranged to assess the effectiveness of the drug at preventing progression of the disease.
The Galahad trial was aimed at men with Prostate Cancer that had spread throughout the body (metastasised) who had specific anomalies in their DNA. Patients took the trial drug Niraparib as a tablet with regular visits to the CRF for blood tests and physical exam to check the effect the drug was having and any possible side effects. Regular imaging scans were arranged to assess the effectiveness of the drug at preventing progression of the disease.The results of this study were published in the Lancet Oncology here.
You can see a list of our current active trials
- Our Team

NIHR Lancashire Clinical Research Facility - Meet the team
CRF Director

Dennis.Hadjiyiannakis@lthtr.nhs.uk
Dr Dennis Hadjiyiannakis, Consultant Oncologist
“I am keen to help investigators across all disciplines to develop clinical research. Particularly, to rise to the challenges demanded by experimental medicine studies.”
 CRF Manager
CRF Manager
RN Jacqueline Bramley
Jacqueline.Bramley@lthtr.nhs.uk
01772 528260
 CRF Senior Research Sister
CRF Senior Research Sister
RN Karen Jones
Karen.Jones4@lthtr.nhs.uk
01772 522031
 CRF Senior Clinical Fellow
CRF Senior Clinical Fellow
Dr David Cameron
David.Cameron@lthtr.nhs.uk
01772 522031“I am inspired by our patient’s generosity in choosing to be involved with clinical research to help mould the future of healthcare”
 Rosalind Szurko
Rosalind Szurko
01772 522031
Rosalind.Szurko@lthtr.nhs.uk CRF Clinical Research Nurse
CRF Clinical Research Nurse
Elizabeth Coates
01772 522031
Elizabeth.Coates@lthtr.nhs.uk
 CRF Assistant Practitioner
CRF Assistant Practitioner
Mathew Anuj
01772 522031
Mathew.Anuj@lthtr.nhs.uk
 CRF Research Facility Administrator
CRF Research Facility Administrator
Colin Davies
01772 522031
Colin.Davies@lthtr.nhs.uk
 EDI Champion
EDI ChampionNita Desai
Nita.Desai@lthtr.nhs.uk
01772 522031“Diversity and Inclusion are about giving value and opportunity to every human being, no matter our differences.”
 PPIE Coordinator
PPIE Coordinator
Calum Reid
Malcolm.Reid@lthtr.nhs.uk - Public and Patient Involvement, Engagement, and Participation (PPIEP)
Every day thousands of patients, carers and the public go the extra mile to help NIHR make research happen. From taking part in clinical trials to working in partnership with researchers, clinicians, social care practitioners and health professionals to improve the quality of our research, how it is designed, delivered and then used to change treatment and care.
- NIHR National Institute of Health and Research statement

We have members of the public involved in all stages of our research projects. This can start from the very beginning of a project, with individuals sharing their life and healthcare experiences to develop the important research questions and make sure they are relevant and important to modern life. We have an active Lay Research Group (LRG) to ensure that we represent the patient and publics opinion, to ensure that research is driven by the community for the community.
The LRG vision is to “build an informed and engaged patient and public membership and to embed members in committees and research teams to advise on how research is planned, conducted and communicated both internally and to the wider community.”
LRG aims to:
- Engage with all areas of the community, especially those who are under-represented, to ensure their voice is heard and embedded in research
- Provide a lived experience perspective to enrich research and to improve participants' experiences
- Improve the quality of research by strengthening the relevance and impact of studies
- Assist in ensuring information is produced in a way that is accessible and easy to understand
- Increase awareness of research within the Trust

If you want to get involved, People in Research is a web-based resource for members of the public who are looking for opportunities to get involved in research. Alternatively visit the NIHR Patients, Carers and the Public website.
- Patients and the Public
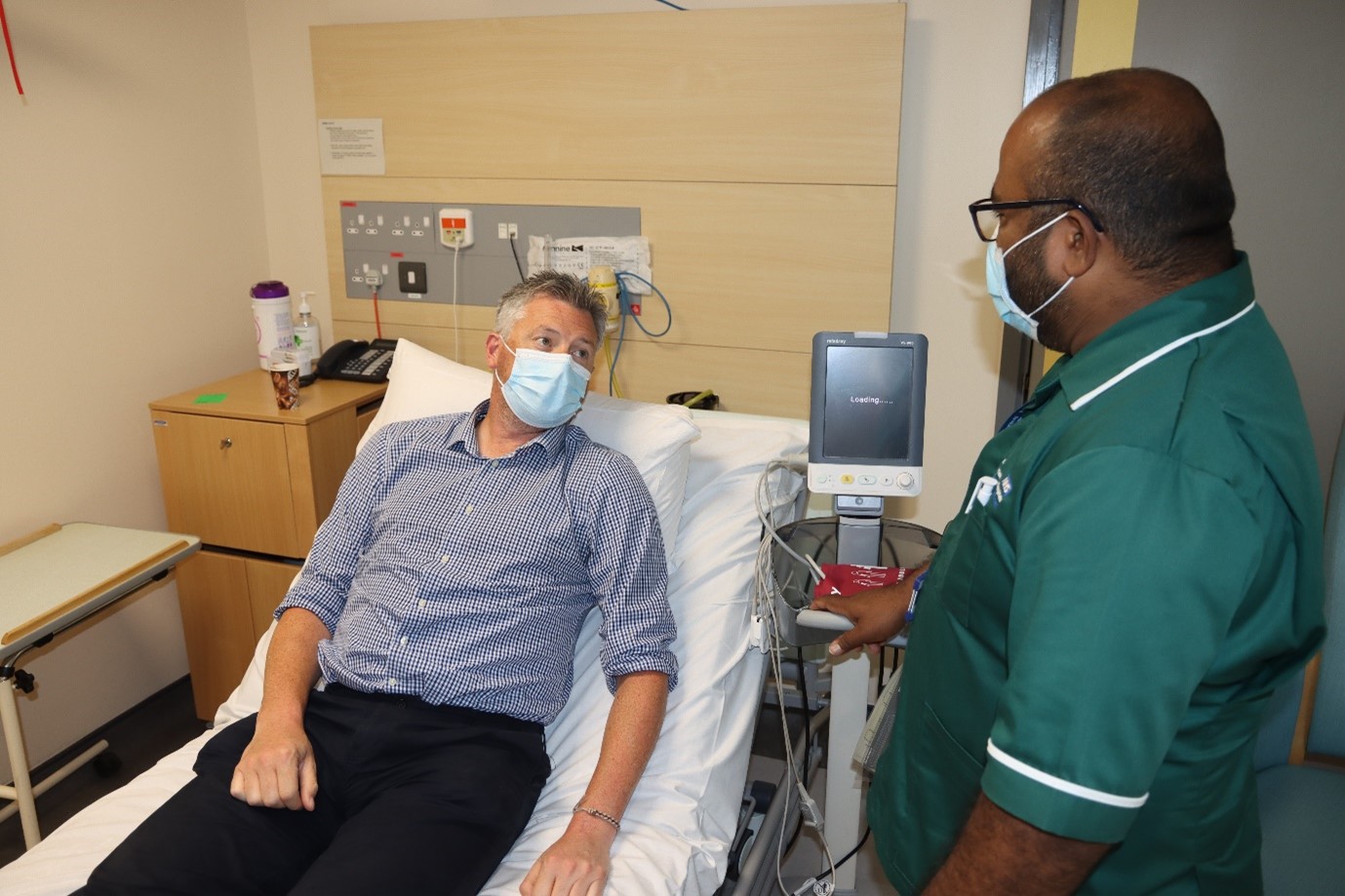
Why do research?
Although there has been huge progress in the development of new treatments and medicines, there is still a lot that we do not know. Research can answer questions, filling in the gaps in knowledge and changing the way doctors and health professionals look after their patients. This means improved treatment and care for you and your family.
Clinical research provides the only reliable evidence for safe, effective healthcare. Without patient and public volunteers that evidence can’t be gained and new treatments cannot be provided.
What is a clinical trial?
A clinical trial is a particular type of research that tests treatments. They are often carried out on a small number of people to begin with, before moving to a larger population. At the NIHR Lancashire CRF we focus on this initial 'early phase' work.
Therefore, clinical trials find out:
- if treatments are safe
- if treatments have any side effects
- if new treatments are better than current standard treatments
- if treatments work to prevent or treat a condition

Trials are being undertaken every day within the NHS and are often managed by hospital doctors and health professionals that may be looking after you.
Who takes part in research trials?
A wide variety of different patients take part in our research studies. This may include male and female, young and old, all ethnic groups and patients with a variety of medical conditions. However, each study looks at something very specific; they will have strict eligibility rules for who is suitable to take part. However, if you are interested in taking part in a clinical trial you can speak to your doctor to see if there are any studies that you may be eligible for.
Do I have to take part?
The simple answer is no. Taking part in research is entirely voluntary. You may be approached by your doctor or nurse and invited to take part. You will be provided with an information sheet and have time to think about the study and ask any questions you wish. If you decide not to take part in a clinical trial your NHS treatment will not be effected and you will continue to be looked after like any other patient. And if you do decide to take part, but change your mind, you can leave the study at any time.
Is it safe?
Clinical trials assess treatments and technologies, whilst other research studies aim to understand health and specific conditions better through testing of new approaches, monitoring or observation. All drugs, including the ones we routinely prescribe to patients, may have side effects. In clinical trials potential risks are carefully balanced against the benefits. Trials are designed to keep risks to a minimum and patients are closely monitored.
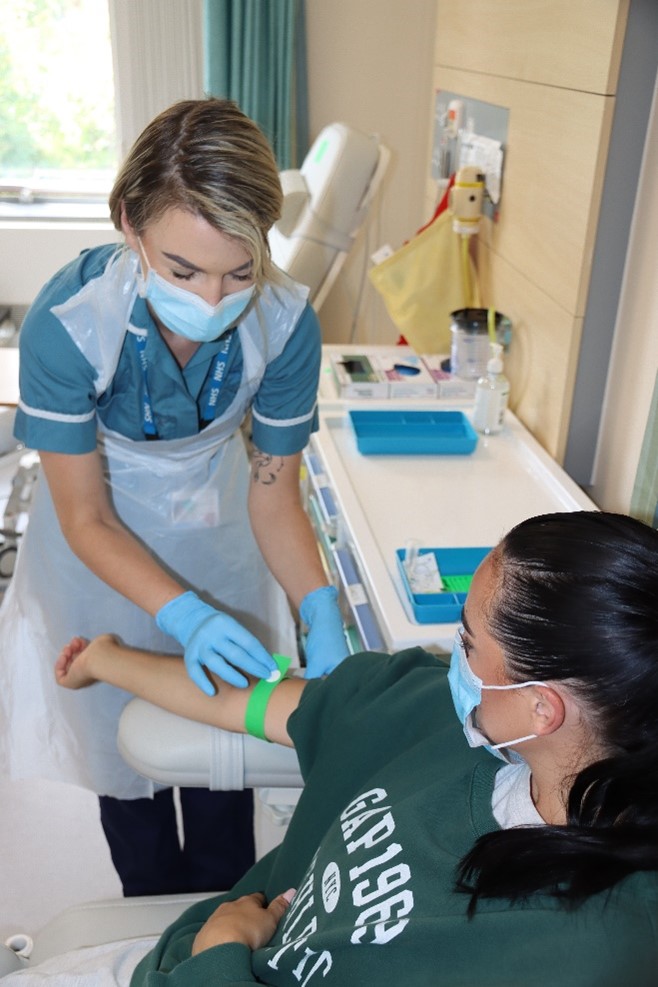
What are the benefits for me?
The reasons people take part in a study vary. Taking part in research is perceived by some people as ‘giving something back’ to benefit others in the future. Other people feel that they personally benefit from the treatment and drugs provided and are reassured by the close medical monitoring and check-ups provided by the researchers.
What will happen to me during a research study?
Every study is different as all research is planned to investigate something specific, meaning the duration of the trial and the investigations required, (e.g. blood sampling, ECG, x-rays) will vary. Therefore it is difficult to say specifically what you may experience during a study. However, bear in mind that all research is carefully planned and you will be provided with more detailed information if you participate in a study.
How will my personal information be used for research?
All NHS organisations (including Health & Social Care in Northern Ireland) are expected to participate and support health and care research. The Health Research Authority set standards for NHS organisations to make sure they protect your privacy and comply with the law when they are involved in research. For further information about how your information may be used can be found here.
Where can I get further information?
If you are considering taking part in research we would encourage you to contact your doctor, health professional or research nurse for further information.
There are also various websites you may like to visit:- www.healthtalkonline.org
Here you can read, watch videos and listen to audio clips about the experiences of people who have already taken part in research. - www.nihr.ac.uk
National Institute for Health Research – where you can find out more about research in the NHS. - www.nhs.uk/conditions/clinical-trials
Here you can find much more information about research and clinical trials as well as links to useful resources. - www.ukctg.nihr.ac.uk
Here you can search for different studies running in the NHS.
Our assurance to you
With research embedded into the fabric and work of the trust, patients can be confident of the same excellent standards of care and safety they expect across our services.
Strong regulation and management
Before it begins, all research involving NHS staff, facilities, service users, patients, their carers or relatives will always have been approved by an independent research ethics committee and other regulatory authorities, and will have been reviewed by the Trust to ensure that it is safe and appropriate to conduct.
- Equality, Diversity and Inclusion (EDI)
We are currently in the process of transferring the EDI 2022 - 2027 Strategy directly onto the page. What you are seeing below is the work in progress.
Equality, Diversity and Inclusion (EDI) Strategy 2022 – 2027
BackgroundThe Centre for Health Research and Innovation (CHRI) and the NIHR Lancashire Clinical Research Facility (LCRF) are based at Royal Preston Hospital and serve the communities within Lancashire and South Cumbria (LSC). We work on the delivery of research trials, including early phase and experimental medicine (EM) studies.We are passionate about offering all communities within LSC access and equal opportunity to be involved in innovative research trials and to ensuring our workforce represents these communities. The areas we serve are very diverse, with areas of significant social deprivation and ill-health.
‘Nearly a third of LSC residents live in some of the most deprived areas across England. The percentage of people living in fuel poverty and unable to afford to heat their homes is higher than the national average: 13% for LSC, the national average is 10.6%.
The percentage of children living in poverty ranges from a low of 12% to as high as 38% in LSC, the national average is 30%.
21,442 people have five or more long term health conditions in LSC. The main causes are cancer, cardiovascular, respiratory, mental health, and neurological conditions. Suicide rates are significantly higher than average in LSC.’
(Equality, Diversity and Inclusion Strategy 2022 – 2027, Lancashire Teaching Hospitals NHS Foundation Trust - LTHTr).
Therefore, ensuring equal access to research studies that have the potential of improving the health and wellbeing of communities is vital.
The LCRF and CHRI are committed to equality, diversity, and inclusion in all we do. This strategy is focused on embedding equality, diversity, and inclusion into the delivery of research within LCRF and CHRI and in our workforce. The strategy will closely follow the EDI strategy of our host NHS Trust, Lancashire Teaching Hospitals NHS Foundation Trust (LTHTr), and the NIHR Equality, Diversity and Inclusion Strategy 2022 -2027 (https://www.nihr.ac.uk/documents/equality-diversity-and- inclusion-strategy-2022-2027/31295?pr=) to ensure collectively we are being ‘consciously inclusive in everything we do for our colleagues and communities’.
Our first strategy has been designed to embed best practice and ensure everything we do is aligned to our vision ‘To be the sustainable hub for the delivery of EM trials to the highest standards in LSC, addressing key clinical problems in priority areas’.
VisionOur Vision is to develop a research department that has EDI at the centre of everything we do; where everybody can access research studies; gives every single member of the team a voice, and empowers them to be whoever they are.This strategy is based on overarching goals: 1) Analysis 2) Training and Development 3) Implementation. The action plan (appendix 1) will be reviewed after 3 years against the SMART objectives identified.
AnalysisTo provide a baseline of our current EDI position, we aim to evaluate and reflect on our EDI maturity using an established maturity model. To do this, we have established an EDI forum (first meeting February 2023) to bring together the knowledge and experiences of staff from the LCRF and CHRI partners. We will work with the Trust and partners EDI Leads to provide leadership, expertise, and direction so we have an accurate understanding of our current position.Alongside the development of access to anonymised patient demographics in the Trusted Research Environment (TRE), we will work with the Trust and partner EDI Leads to collate workforce baseline data to understand the differences and disparities within the team.
We will conduct an audit to provide us with data on the communities we are involving in research. This will include current and previous patient data. This will provide us with a baseline to understand if there are disparities with the patients accessing research studies.
Training and DevelopmentWe will appoint a Coordinator with CHRI to oversee day to day aspects of EDI alongside Patient and Public involvement, Engagement and Participation (PPIEP). Working with this person we will develop our understanding and knowledge on EDI issues, barriers, and implementation through having research representation on LTHTr Ambassador Groups.On an annual basis we will invite colleagues to research huddles to educate us on the lived experience of a wide variety of characteristics. This will educate the team on appropriate terminology to ensure no colleague or patient is unintentionally excluded due to inaccuracies in the way documents are written, and staff communicate. These colleagues will also be invited to the EDI forum so their points of view and experience will inform future work.
We will work with the NIHR Manchester BRC EDI leads and attend EDI training offered.
We will ensure all members of the research team have undertaken LTHTr EDI eLearning resources available on the Trust internet.
We will be actively involved in work being undertaken by the LTHTr research intern and ensure the learning is implemented within the department. We will encourage all staff members within the LCRF and CHRI to complete the Northwest Research Workforce Survey. This survey has been designed to gather data on career progression and access to non-mandatory training and Continued Professional Development to understand any barriers staff face with accessing training and career progression.
ImplementationWe will ensure all strategies and policies written by the LCRF and CHRI have an EDI section to support an increased momentum and collective focus for improvement.We will aim for strategies, policies, and procedures to be reviewed by minority groups through LTHTr colleague Ambassador Forums and Patient Involvement Groups. Additionally, we aim to implement the use of Equality Impact Assessments at the initiation of new projects and policies to ensure that barriers and under- representation are identified and can be mitigated.
We will include EDI in the cycle of business for the LCRF Operational Group meetings to ensure local actions are being taken to progress the EDI agenda.
We aim to review policies and standard operating procedures to ensure they are consciously inclusive: for example to ensure they are gender, culture, disability and LGBTQ+ inclusive.
We will ensure all research job titles, job descriptions, and person specifications are EDI-compliant.
We will ensure all communications, publications, patient facing leaflets, and internal colleague information is gender-inclusive by changing references to gender-specific roles to gender-neutral terminology; specifically using terms such as parent/guardian, you/their/them, people or individuals, siblings, humankind (not mankind), artificial/synthetic (not manmade).
We seek to collect the demographics of patients for all 9 protected characteristics. They will be subject to audit to ensure that any risk of discrimination is monitored.
Following our initial patient audit, we will continue to routinely monitor minority characteristics of our patients every year.
We will ensure the LCRF and CHRI have a completed Supporting Disability in the Workplace Agreement with every colleague who has a disability or long-term condition and requests one. We will ensure these are regularly reviewed and updated.
We ensure all assistive technology/reasonable adjustments meet the needs and standards of our disabled staff working in the research department making sure they are correctly resourced, funded and arrive promptly.
We will seek to recruit public members involved in the research process from a diverse background that is representative of the communities within LSC.
We will take steps to increase the representation of minority colleagues within the research team to ensure the team is broadly representative of the communities we serve. This includes reviewing our recruitment processes, ensuring diverse interview panels for all interviews, and including EDI questions within the interviews.
Through undertaking an audit, we will seek to understand disparities in performance management in colleagues with protected characteristics working within the research department. Specifically this will be in relation to formal performance management processes, appraisal ratings, talent management ratings, and ability to access training and development opportunities beyond mandatory training.
We will report completion of the EDI eLearning resources to relevant groups and committees and ensure we reach a completion rate of 100%. We will include this training within the new staff induction to the department.
Using the results of the Northwest Workforce Survey we will ensure managers are giving all staff equal access to training and addressing barriers to accessing training and career progression within 1:1 meetings.
How we will measure and oversee progressImplementation of this strategy will be shared to managers within the LCRF and CHRI and the EDI Forum.The Equality, Diversity and Inclusion Forum will:
- Be responsible for recommending approaches to EDI implementation
- Review progress against the objectives within this strategy (Appendix 1)
- Engage with Ambassador Groups within the Trust and disseminate shared learning
- Share and celebrate examples of improvements and changes made as a result of implementations of the overarching goals
Appendix 1: LCRF EDI SMART Objectives (to be formally reviewed and evaluated against SMART objectives after 3 years)
Overarching Goal 1 - AnalysisGoal
Specific
Measurable
Attainable
Relevant
Time-bound
Task Owner
Overall objective
How specifically will we address this goal
How will success be measured
Is this objective achievable with current resources?
Is the objective relevant to the overarching
goal
Within what timeline must the outcomes be achieved and measured.
Who is the principal lead for achievement of this objective.
Establish a diverse EDI forum involving EDI leads and experts from the Trust and partners to reflect Trust and NIHR policy/strategy. EDI to report to the R&I Committee
Identify named EDI leads across the Trust and partners.
Organise and host an EDI specific forum in person and/or remotely once every
4 months.
Documented minutes of EDI forum meetings as evidence of outcomes.
Forum review and comment on LCRF policies to reflect Trust/NIHR strategy.
Yes
Yes
April 2023
LCRF Manager
Evaluate initial EDI maturity within the LCRF and CHRI to establish a baseline
Forum members to evaluate EDI maturity level model and agree upon most appropriate
description.
Documented minutes produced by EDI Forum chair documenting forum consensus on baseline EDI
maturity
Yes
Yes
March 2024
EDI Forum
Identify steps to develop EDI maturity
EDI Forum to review EDI maturity model, agree a realistic target maturity level for the duration of the
current period.
Documented report or minutes from the EDI Forum chair presenting SMART Objectives for achieving the
target maturity level.
Yes
Yes
March 2024
EDI Forum
Continue to monitor EDI maturity and adapt/direct EDI activities to develop EDI maturity
EDI Forum to routinely assess the progress of SMART objectives produced to progress the
EDI maturity level
Documented minutes from EDI Forum Chair addressing planned SMART objectives and progress made
Yes
Yes
March 2025
EDI Forum
Evertyhing above this line is the unfinished, ongoing work of the transferring of the contents of the EDI PDF file directly onto the page.
“We are committed to equality, diversity and inclusion in everything we do. Diverse people and communities shape our research, and we strive to make opportunities to participate in research an integral part of everyone’s experience of health and social care services. We develop researchers from multiple disciplines, specialisms, geographies and backgrounds, and work to address barriers to career progression arising from characteristics such as sex, race or disability.” – NIHR

Equality, Diversity, and Inclusion is about giving value and opportunity to every human being, no matter our differences. It is essential that the research we conduct at the NIHR Lancashire CRF is relevant to the communities that we serve, and that we accurately reflect the makeup of local populations in our patient groups.

Our goals
In order to make sure that we are fairly and accurately representing individual patients as well as our local communities, we are aiming to:
- Work with our communities to determine their areas of interest for the direction of research
- Strengthen our partnerships with organisations that can bring different perspectives, to maximise shared opportunities
- Design an educational awareness programme
- Widen the diversity of patient participation in our CRF activities and studies
- Understand the needs of our population and work with them to design and deliver services that meet these needs
- Ensure equal opportunities for all, regardless of an individual’s characteristics
- Help improve access, experiences and health outcomes for all patients and communities
- Actively promote equality, diversity, and inclusion by creating the conditions that enable compassion and inclusivity to thrive
- Industry and Partners
Industry
Why collaborate with us?
Serving a population of 1.8 million and providing a range of care in both a secondary and tertiary healthcare setting, Lancashire Teaching Hospitals NHS Foundation Trust is well placed to make a real impact on the health of the region through evidence based practice.
The NIHR Lancashire CRF benefits from its close proximity to a number of quality academic institutions as well as being a provider of a number of specialist services across the region.
Research in the NHS is a perpetually evolving landscape yet the key role it has to play in the future of the NHS is clear. The NHS Constitution cites that ‘the NHS aspires to the highest standards of excellence and professionalism…through its commitment to innovation and to the promotion and conduct of research to improve the current and future health and care of the population’.
The NIHR Lancashire CRF has produced a library of standard operating procedures (SOPs). These SOPs have been created for those undertaking research within Lancashire Teaching Hospitals NHS Foundation Trust in order to provide clear guidance on the conduct of a study.
Partnering with us gives you:
- Rapid trial feasibility, start up and completion – you will have a dedicated Project Manager from the Research Access Team who will be you main point of contact during the set-up / feasibility process
- Access to experienced clinical investigators across secondary and tertiary healthcare
- Well-experienced research delivery team, made up of research nurses, clinical trial support officers and administrators
- Wide and varied patient population with diverse healthcare needs
- We have strong links with all NIHR Clinical Research Networks
- Well established partnerships with local higher educational partners and collaborators
- Successful recruitment to our clinical trials
Case study: MyPad (Dr Christian DeGoede)
Read the "MyPad" research paper

The LCRF has supported the recruitment and delivery of over 16 trials in neurology. Supporting a LTHTr Consultant Paediatric Neurologist, Dr Christian DeGoede, the LCRF has delivered the MyPad study in collaboration with the School of Engineering at the University of Central Lancashire (UCLAN). The study, trialling an Intelligent Bladder Pre-void Alerting System, recruited healthy children in the LCRF to gain information about the comfort and durability of the device. This is one of our first studies to run over a weekend period and would not have been possible without the facility and the NIHR funding.
Our research partners and affiliate organisations
Lancashire & South Cumbria NHS Foundation Trust
Lancaster University
https://www.lancaster.ac.uk/research/
University of Central Lancashire (UCLAN)https://www.uclan.ac.uk/research
NIHR
Rosemere Cancer Foundation
Blackpool Teaching Hospitals NHS Foundation Trust - Publications
This database contains details of the research and publications outputs of staff and students at Lancashire Teaching Hospitals NHS Foundation Trust.
The database was launched in August 2019 and currently holds information about research published from 2015 to the present.
If you have any questions please contact library@lthtr.nhs.uk
Are you publishing research conducted in the NIHR Lancashire CRF or supported by NIHR funded research teams? If so please use this NIHR acknowledgment wording. Failure to comply could mean financial penalties for the Trust.
The most recent publications crediting the Lancashire CRF:
- Morris AD, Morais CLM, Lima KMG, Freitas DLD, Brady ME, Dhaygude AP, et al. A comparative analysis of different biofluids using Raman spectroscopy to determine disease activity in ANCA-associated vasculitis. Journal of Biophotonics. 2021;14(4).
LINK: https://pubmed.ncbi.nlm.nih.gov/33242117/
- Wu HHL, Van Mierlo R, McLauchlan G, Challen K, Mitra S, Dhaygude AP, et al. Prognostic performance of clinical assessment tools following hip fracture in patients with chronic kidney disease. Int Urol Nephrol. 2021:1-9.
LINK: https://pubmed.ncbi.nlm.nih.gov/33686533/
- Hill J, Hare M. Statins used for secondary prevention in patients with stroke reduce the risk of further ischaemic strokes and cardiovascular events. Evid Based Nurs. 2021;24(1):24.
LINK: https://pubmed.ncbi.nlm.nih.gov/31874941/
- Nixon AC, Brown J, Brotherton A, Harrison M, Todd J, Brannigan D, et al. Implementation of a frailty screening programme and Geriatric Assessment Service in a nephrology centre: a quality improvement project. J Nephrol. 2020.
LINK: https://pubmed.ncbi.nlm.nih.gov/33040293/
- Nixon AC, Bampouras TM, Gooch HJ, Young HML, Finlayson KW, Pendleton N, et al. The EX-FRAIL CKD trial: a study protocol for a pilot randomised controlled trial of a home-based EXercise programme for pre-frail and FRAIL, older adults with Chronic Kidney Disease. Bmj Open. 2020;10(6).
LINK: https://pubmed.ncbi.nlm.nih.gov/32571859/
- Kuru K, Ansell D, Jones M, Watkinson BJ, Caswell N, Leather P, et al. Intelligent autonomous treatment of bedwetting using non-invasive wearable advanced mechatronics systems and MEMS sensors Intelligent autonomous bladder monitoring to treat NE. Medical & Biological Engineering & Computing. 2020;58(5):943-65.
LINK: https://pubmed.ncbi.nlm.nih.gov/32090271/
- D. Hadjiyiannakis, D. Dimitroyannis, L. Eastlake, C. Peedell, L. Tripathi, R. Simcock, A. Vyas, E. Deutsch, A.J. Chalmers, Personal View: Low-Dose Lung Radiotherapy Should be Evaluated as a Treatment for Severe COVID-19 Lung Disease. Clinical Oncology Volume 33, Issue 1 2021, Pages e64-e68.
LINK: https://pubmed.ncbi.nlm.nih.gov/32829986/
- Morris AD, Morais CLM, Lima KMG, Freitas DLD, Brady ME, Dhaygude AP, et al. A comparative analysis of different biofluids using Raman spectroscopy to determine disease activity in ANCA-associated vasculitis. Journal of Biophotonics. 2021;14(4).
- Our Facility
NIHR Lancashire Clinical Research Facility's opening hours are 8am to 6pm Monday to Friday
Inpatient and Outpatient
We have one inpatient room, and three outpatient rooms.
The inpatient room offers the following:
.jpg)
- A full size standard bed providing comfort for longer stays.
- An integrated ceiling hoist leading to a wet room.
- TV access providing entertainment.
The outpatient rooms offer the following:
.jpeg)
- One consulting room
- Two couch flexible space which can be modified to accommodate a variety of research studies
- Venepuncture room
Additional Facilities
.jpeg)
In addition to the clinical rooms we have:
- Clinical laboratory for sample processing and short term storage of samples
- Office space in the multi-user room for research teams using the facility
- Meeting room with teleconferencing facilities
- Two bookable meeting rooms
- Large secure filing room to house sensitive research documentation and files
- Contact us
Patients
If you are a patient keen to be involved with Research at Lancashire CRF speak to your disease specialist, contact 01772 522031 or LancashireCRF@lthtr.nhs.uk
Investigators
We would be delighted to discuss what we can offer to support your clinical trial. Please contact Research.Access@lthtr.nhs.uk
Sponsors
For industry Sponsors looking for clinical Research sites please contact the Lancashire Teaching Hospitals Research Access Team at Research.Access@lthtr.nhs.uk
- Make a donation
We are extremely grateful for any donations received to further the work performed at the Lancashire CRF. If you would like to support the work we do, please visit our dedicated Lancashire Teaching Hospitals Charity site.
Please make it clear when you donate to the charity that you would like your funds to go to the NIHR Lancashire Clinical Research Facility.
If you have any queries about making a donation, please contact the CRF Manager.
Current Funding Goals
Clinical Educator

A Clinical Educator covers aspects of specialist training required for increasingly advanced clinical trials.
A Point-of-care (POC) Urinalysis Machine

Electronic urinalysis machines allow for rapid, accurate and repeatable results for what is an important (if rarely celebrated) aspect of many clinical trials.
- Useful links
- www.healthtalkonline.org
Here you can read, watch videos and listen to audio clips about the experiences of people who have already taken part in research. - www.nihr.ac.uk
- National Institute for Health Research – where you can find out more about research in the NHS.
- www.nhs.uk/conditions/clinical-trials
Here you can find much more information about research and clinical trials as well as links to useful resources. - www.ukctg.nihr.ac.uk
Here you can search for different studies running in the NHS. - INVOLVE – Promoting public involvement in NHS, public health and social care research (http://www.invo.org.uk/)
- People in Research a web-based resource for members of the public who are looking for opportunities to get involved in research.
-
https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial?utm_source=ecmcwebsite
- www.healthtalkonline.org
- Relevant Leaflets / Documents / Links
- How to find us
Our address is:
NIHR Lancashire Clinical Research Facility
Avondale Unit,
Royal Preston Hospital,
Sharoe Green Lane,
Fulwood,
PR2 9HT















